| 1.
| In step 9, ammonium chloride (NH4Cl) was added to lower the blood pH. The ammonium ion is what acts as the acid. The chloride ion does not have any acid/base properties.
a. Why is the ammonium ion used as the acid source instead of HCl?
b. Why is NH4Cl used instead of some other ammonium compound (like NH4NO3 or NH4I)?
|
| 2.
| What observation did you make each time an acidic substance was added to the beaker? Write a general reaction OR use equilibrium arguments to explain this.
|
| 3.
| The ability of hemoglobin (Hb) to carry oxygen throughout the body as oxyhemoglobin (HbO2) is dependent on the pH of the blood. What effect would acidosis have on the ability of a patient to transport oxygen?
HbH+ + O2 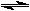 HbO2 + H+ HbO2 + H+
|
| 4.
| The solution on the left was made by dissolving several drops of blood in some water. The solution on the right was made the same way except that a small amount of HCl was also added to this tube. Based on your general knowledge about the color of blood and the information in question 3, propose an explanation for what happened.

|
| 5.
| A fresh sample of soda had a pH of 2.92. The soda was placed on a magnetic stirrer and made to go flat. The pH is measured again. Should the pH of the flat soda be higher, lower or the same as the pH of the fresh soda?
|
| 6.
| The bicarbonate/carbonic acid buffer is also present in chickens. However, chickens also combine the carbonate in their blood with calcium ions to make calcium carbonate for their eggshells. Since chickens do not sweat, they pant in hot weather. What effect would this have on the pH of their blood and the strength of the eggshells they produce?
2 H2O + CO2 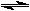 H2CO3 + H2O H2CO3 + H2O 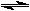 H3O+ + HCO3- H3O+ + HCO3- 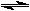 CO3-2 + H3O+ CO3-2 + H3O+
|
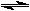 HbO2 + H+
HbO2 + H+
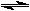 H2CO3 + H2O
H2CO3 + H2O 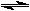 H3O+ + HCO3-
H3O+ + HCO3- 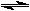 CO3-2 + H3O+
CO3-2 + H3O+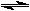 HbO2 + H+
HbO2 + H+
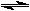 H2CO3 + H2O
H2CO3 + H2O 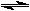 H3O+ + HCO3-
H3O+ + HCO3- 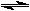 CO3-2 + H3O+
CO3-2 + H3O+


