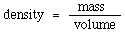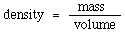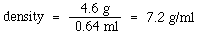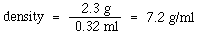Density - Background
The density of a substance is defined as the amount of matter contained in a given volume of the substance. Numerically, this can be expressed as the mass per unit volume and is calculated using the formula:
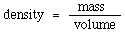
While any units of mass and volume can be used to calculate density the most common are grams (g) and milliliters (ml). This gives density the units of grams per milliliter (g/ml).
An example: A 4.6 g piece of zinc is determined to have a volume of 0.64 ml. What is the density?
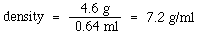
Density is an extensive property of a substance. This means that the density is not dependent on the amount of material examined. Suppose that the piece of zinc in the example above were cut precisely in half. The mass would now be 2.3 g and it would have a volume of 0.32 ml. The density would therefore be:
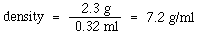
Notice that the value of the density did not change when a smaller piece of the zinc was examined. Interestingly, mass and volume are both intensive properties. Their values change when the amount of material examined changes.
Continue on and read about determining the mass of an object.