Equilibrium and LeChatelier's Principle - Background
Most of the reactions you have encountered so far proceed in only one direction. That is, when the reaction has stopped all of the reactants have been converted into products. This type of reaction is said to go to completion and occurs when the products of the reaction are much more stable than the reactants. This is not true of all reactions. Sometimes the products react with each other to reform the reactants. The reaction of the reactants to form the products is called the forward reaction. The reaction of the products to form the reactants is called the reverse reaction.
Consider the reaction of hydrogen gas with iodine vapor to form hydrogen iodide. The forward reaction is:
H2 + I2 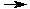 2 HI
2 HI
while the reverse reaction is:
2 HI 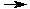 H2 + I2
H2 + I2
For convenience these two reactions are usually written together with a special arrow between them.
H2 + I2 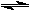 2 HI
2 HI
Continue with more background on equilibrium.
 2 HI
2 HI
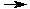 2 HI
2 HI
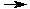 H2 + I2
H2 + I2
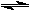 2 HI
2 HI



