| Name | Functional Group Structure | Example Compound | Official Name of Example (Common Name) Formal Name Ending |
| ketone | 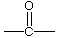
| 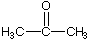
| 2-propanone (acetone or dimethyl ketone) -one endings |
| aldehyde | 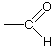
| 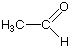
| ethanal (acetaldehyde) -al endings |
Aldehydes and Ketones |
| Name | Functional Group Structure | Example Compound | Official Name of Example (Common Name) Formal Name Ending |
| ketone | 
| 
| 2-propanone (acetone or dimethyl ketone) -one endings |
| aldehyde | 
| 
| ethanal (acetaldehyde) -al endings |
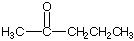 is 2-pentanone
is 2-pentanone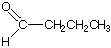 is butanal.
is butanal.




| introduction | background | prelab | experiment | postlab |