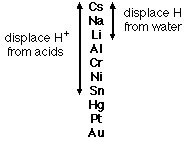
Metal Activity Series |

2 Al(s) + 6 HCl(aq)  3 H2(g) + 2 AlCl3(aq) 3 H2(g) + 2 AlCl3(aq)
| (molecular equation)
|
2 Al(s) + 6 H+(aq)  3 H2(g) + 2 Al+3(aq) 3 H2(g) + 2 Al+3(aq)
| (net ionic equation) |
2 Al(s) + 3 Hg(NO3)2(aq)  3 Hg(l) + 2 Al(NO3)3(aq) 3 Hg(l) + 2 Al(NO3)3(aq)
| (molecular equation)
|
2 Al(s) + 3 Hg+2(aq) 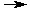 3 Hg(l) + 2 Al+3(aq) 3 Hg(l) + 2 Al+3(aq)
| (net ionic equation) |




| introduction | background | prelab | experiment | postlab |