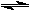 2 HI
2 HI
LeChatelier's Principle - Observable Changes |
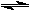 2 HI
2 HI
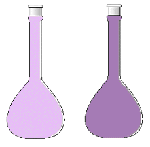
| applied stress | direction of shift | effect on [H2] | effect on [I2] | effect on [HI] | observed color change |
| temperature raised | right | decreased | decreased | increased | lighter purple |
| H2 removed | left | decreased | increased | decreased | darker purple |
| I2 removed | left | increased | decreased | decreased | lighter purple |




| introduction | background | prelab | experiment | postlab |